“We’re building an Africa that’s in good health.”
Our innovations have helped 400,000 patients make savings on high quality medicines.
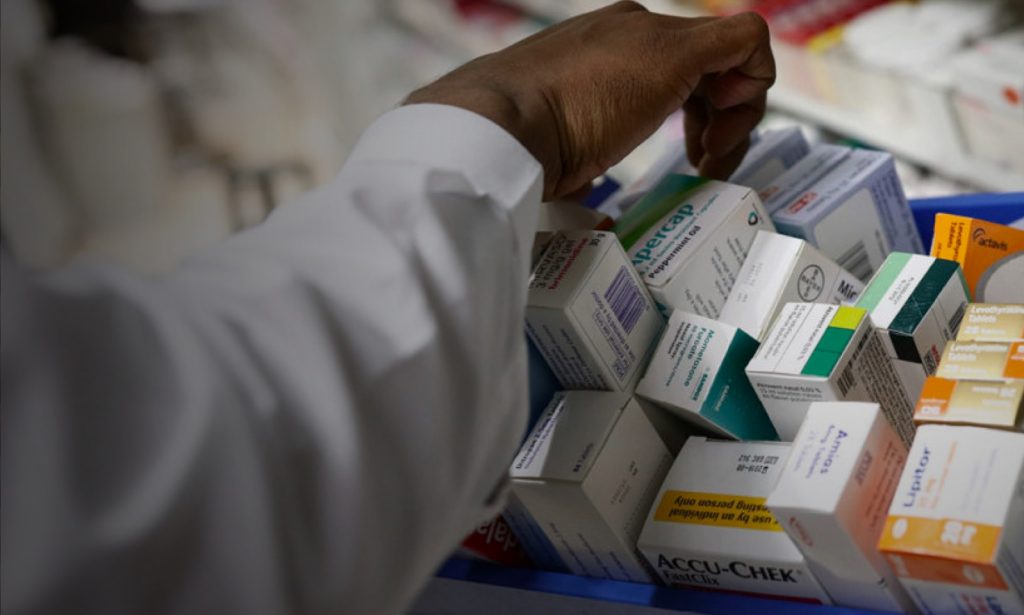
For pharmacies, it meansthe drugs your patients need are always in stock

For patients, it meansyou can get your prescription, even when you’re short

For partners, it meansyou can reach every African who needs medicine
Meet Mutti
The easy pay scheme for patients that guarantees the medicines you need are always available, always affordable.
Get your Mutti cardmPharma wins
 award
award
The Skoll award recognises innovation by a social enterprise.
mPharma is overturning the injustice of millions of people who currently lack access to affordable medicines.
To win this award, we demonstrated a compelling plan and a collaborative approach to achieving impact across Africa.
9
African countries
155
Hospital partners
850
Pharmacies and Drug stores
2M
Patients and growing